Mouse IFN-Alpha All Subtype ELISA Kit, High Sensitivity (Serum, Plasma, TCM)
Catalog Number: 42115
This Mouse Interferon Alpha ELISA has an LLOQ of 2.38 pg/ml and can detect all 14 mouse IFN-Alpha subtypes in serum, plasma, and tissue culture media. This ELISA provides total IFN-Alpha readout in approximately 2 hours.
Product Name: VeriKine-HS Mouse Interferon-Alpha All-Subtype ELISA Kit (Serum, Plasma, TCM)
$695.00
Product Info
| Matrix Compatibility | Serum, Plasma, Tissue Culture Media |
|---|---|
| Assay Range | 2.38 - 152 pg/ml |
| LLOQ |
2.38 pg/ml Need more sensitivity? Check out our Sample Testing Services |
| Assay Length | 1 hour, 54 minutes |
| Specificity | All 14 Mouse IFN-α subtypes |
| CVs | Inter-Assay CV: ≤ 10% Intra-Assay CV: ≤ 8% |
The VeriKine-HS Mouse IFN-Alpha ELISA kit has been developed to quantitate low levels of IFN-Alpha in mouse serum, plasma and cell/tissue culture media (10% FBS) using a sandwich immunoassay. The kit contains an anti-mouse IFN-Alpha detection antibody conjugated to biotin and quantified by streptavidin horseradish peroxidase (HRP) using tetramethyl-benzidine (TMB) as a substrate.
The kit standard is mouse IFN-Alpha 4 protein. It is important to run the standard curve in the same matrix as one’s samples unless one has demonstrated that the matrix does not significantly affect the signal.
Specifications
| CVs and Spike Recovery |
Inter-Assay CV ≤ 10%
Spike Recovery ≥ 80% in Serum |
|---|---|
| Cross-reactivity |
Very weak cross-reactivity detected with:
No cross-reactivity with:
|
| Synonyms | IFN alpha, IFN-Alpha, Multisubtype Interferon Alpha, alpha interferon, Type I interferon, IFN-α |
| Storage | 2-8°C |
| Expiration Date | One year from the date of manufacture |
| Shipping Condition | Wet Ice |
Materials Provided:
- Pre-coated microtiter plate
- Plate Sealers
- Wash Solution Concentrate
- Mouse IFN-Alpha 4 Standard, 10,000 pg/ml
- Sample Diluent
- Antibody Concentrate
- Antibody Diluent
- HRP Conjugate Concentrate
- HRP Diluent
- TMB Substrate Solution
- Stop Solution
Additional Materials Required (Not Provided)
- Microplate reader capable of reading an OD at a wavelength of 450 nm
- Variable volume microtiter pipettes
- Adjustable multichannel pipette (50-300 μl)
- Reagent reservoirs
- Wash bottle or plate washing system
- Distilled or deionized water
- Serological pipettes (1, 5, 10 or 25 ml)
- Disposable pipette tips (polypropylene)
- Plate shaker
Tech Info & Data
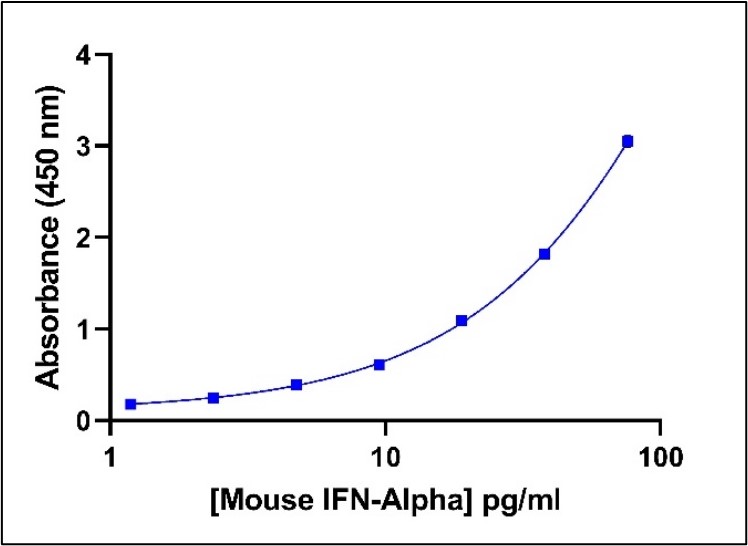
Representative Mouse IFN-Alpha Standard Curve in Sample Diluent
Intra and Inter-Assay CVs to measure Precision |
|||
| Concentration (pg/ml) | Endogenous Level | ||
|---|---|---|---|
| Low | Medium | High | |
| Intra-Assay CV (%) | 4.4 | 3.3 | 2.8 |
| Inter-Assay CV (%) | 7.8 | 5.8 | 8.7 |
Spike Recovery |
|||
| Dilution | Average Concentration (pg/ml) | % Linear Adjusted Dilution | |
|---|---|---|---|
| Not Adjusted for Dilution | Adjusted for Dilution | ||
| 2 | 39.5 | 79.1 | - |
| 4 | 19.1 | 76.6 | 96.8 |
| 8 | 9.7 | 77.3 | 97.8 |
| 16 | 4.9 | 78.8 | 99.7 |
| 32 | 2.5 | 80.7 | 102.0 |
| 64 | 1.2 | 77.7 | 98.2 |
| Avg | - | 78.4 | 98.9 |
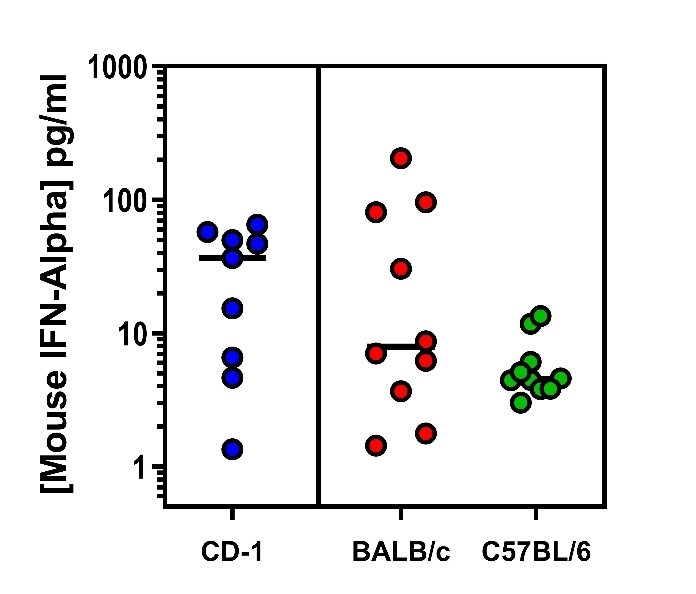
Endogenous Mouse IFN-Alpha Quantified in Plasma or Serum From Three Mouse Strains Using PBL’s High Sensitivity Mouse IFN-Alpha All Subtype ELISA
Background
Interferons (IFNs) are an extensive set of cytokines exhibiting pleiotropic activities and playing major roles in both innate and adaptive immunity. The Type I IFNs genes consist of multiple IFN-Alpha genes, at least one IFN-Beta gene in most vertebrates, and a few other family members including limitin in the mouse and IFN-Omega and -Epsilon in mice and humans. In the mouse, fourteen (14) IFN-Alpha genes have been cloned which exhibit at least 75% identity at the protein level. In the therapeutic setting, IFN-Alpha is used to treat viral diseases and certain cancers and is therefore frequently studied in mouse and other animal models of these diseases. Several lines of evidence indicate that IFN-Alpha proteins may be involved in the development of specific autoimmune diseases and that treatment with IFN-Alpha blocking antibodies may comprise a useful therapeutic strategy.
The expression of interferon genes (and therefore, proteins) is regulated by a group of interferon regulatory factors, i.e., the IRF family of proteins. Cellular levels of IRF3 and IRF7 regulate the expression of individual IFN-Alpha subtypes with most IFN-Alpha subtype genes requiring IRF7 for expression. IRF7 protein is either constitutively expressed or inducible, depending on cell type. In certain mouse cell lines, IRF7 expression is induced in particular by at least IFN-Beta and IFN-Alpha4 which then leads to expression of the other IFN-Alpha subtype genes. Additionally, murine IFN-Beta may be requisite for fibroblast synthesis of IFN-α but may not be required for other cells’ synthesis of IFN-Alpha. The IRF3/IRF7 signaling cascade is a focal point for initial and progressive cellular responses to pathogens where hundreds of genes are subsequently regulated in a temporally coordinated cascade.
Plasmacytoid dendritic cells (PDCs) are highly efficient producers of IFN-Alpha, but a wide variety of cells can also produce lower levels of these protein subtypes. The particular IFN-Alpha subtype genes that are expressed appear to be somewhat cell and stimulus-specific and, based on the results published from several gene induction studies, the time courses of induction of the individual protein subtypes are expected to differ markedly.
Citations
15 Citations:
- Tong et al., (2023). "Nucleotide modifications enable rational design of TLR7-selective ligands by blocking RNase cleavage", J. Exp Med., 221(2):e20230341, PMID: 38095631, DOI: 10.1084/jem.20230341 (link)
- Kerzel, T. et.al., (2023), "In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases", Cancer Cell, S1535-6108(23)00347-1, PMID: 37863068, DOI: 10.1016/j.ccell.2023.09.014 (link)
- Uccello, T.P., et al., (2023), "New insights into the responder/nonresponder divide in rectal cancer: Damage-induced Type I IFNs dictate treatment efficacy and can be targeted to enhance radiotherapy", Cell death Dis., 14(7):470, PMID: 37495596, DOI: 10.1038/s41419-023-05999-3 (link)
- Liu, H. et al., (2023), "Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy", JCI Insight, 8(12):e168945, PMID: 37345657, DOI: 10.1171/jci.insight.168945 (link)
- Ramirez-Valdez, R.A. et al., (2023), "Intravenous heterologous prime-boost vaccination activates innate and adaptive immunity to promote tumor regression", Cell Rep. 42(6):112599, PMID: 37279110, DOI: 10.1016/j.celrep.2023.112599 (link)
- Li, N. et al., (2023), "STING controls opioidinduced itch and chronic itch via spinal tank-binding kinase 1-dependent type I interferon response in mice", J. Neuroinflammation, 20(1):101, PMID: 37122031, DOI: 10.1186/s12974-023-02783-0 (link)
- Preston, S.P. et al., (2023), "A necroptosis-independent function of RIPK3 promotes immune dysfunction and prevents control of chronic LCMV infection", Cell Death Dis. 14(2):123, PMID: 36792599, DOI: 10.1038/s41419-023-05635-0 (link)
- Gunne, S. et al., (2023), "TMPRSS2 Impacts Cytokine Expression in Murine Dendritic Cells", biomedicines, 11:419, DOI: 10.3390/biomedicines11020419 (link)
- Kwart, D. et al., (2022), "Cancer cell-derived type I interferons instruct tumor monocyte polarization", Cell Rep., 41(10):111769, PMID: 36476866, DOI:10.1016/j.celrep.2022.111769 (link)
- Kang, T. G. et al., (2022), "Viral coinfection promotes tuberculosis immunopathogenesis by type I IFN signaling-dependent impediment of Th1 cell pulmonary influx", Nat. Commun. 13(1):3155, PMID: 35672321, DOI: 10.1038/s41467-022-30914-3 (link)
- Choi, J, H. et al., (2020), "DUSP11-mediated control of 5'-triphosphate RNA regulates RIG-I sensitivity", Genes & Development, 34:1, PMID: 33184222, DOI: 10.1101/gad.340604.120 (link)
- Tang, X, et al. (2020). Sustained IFN-I stimulation impairs MAIT cell responses to bacteria by inducing IL-10 during chronic HIV-1 infection. Science Advances, 13 pgs. PMID: 32128419. (link)
- Leylek, Rebecca, et al. (2019). Integrated Cross-Species Analysis Identifies a Conserved Transitional Dendritic Cell Population. Cell Reports, 39 pgs. PMID: 31825848. (link)
- Gale, Emily, et al. (2019). A Nanoparticle Platform for Improved Potency, Stability, and Adjuvanticity of Poly(I:C). Advanced Therapeutics, 6 pgs. PMID: no PMID. (link)
- Hickerson, Brady, et al. (2020). Type I interferon underlies severe disease associated with Junín virus infection in mice. eLife. PMID: no PMID. (link)
Background Literature:
- Krause CD, Pestka S. (2005) “Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives,” Pharmacol Ther. 106(3):299-346.
- Chevaliez, S, Pawlotsky, JM (2009); “Interferons and their use in persistent viral Infections”; in Handbook of Exp Pharmacol; Vol.189; 203-41.
- Antonelli, G. (2008); “Biological basis for a proper clinical application of alpha interferons”; in New Microbiologica.; Vol. 31; Issue 3; 305-318.
- Ascierto, PA, Kirkwood, JM (2008); “Adjuvant therapy of melanoma with interferon: lessons of the past decade”; in Journal of Transl Med; Vol. 27; 60:62.
- Ferrantini, M, Capone, I, Belardelli, F (2007) “Interferon alpha and cancer: mechanisms of action and new perspectives of clinical use” in Biochimie.; Vol. 89; Issues 6 and 7; 884-893.
- Fleischmann, WR Jr, Wu, TG (2005); “Development of an interferon-based cancer vaccine protocol: application to several types of murine cancers”; in Methods Mol Med.; Vol. 116; 151-66.
- Meyer, O (2009); “Interferons and autoimmune disorders” in Joint Bone Spine. Vol. 76; Issue 5; 464-73.
- Burdick, LM, Somani, N, Somani, AK (2009); “Type I IFNs and their role in the development of autoimmune diseases”; in Expert Opinion Drug Safety; Vol. 8; Issue 4; 459-72.
- Kötter, I, Hamuryudan, V, Oztürk ,ZE, Yazici, H (Jan 7, 2010); “Interferon therapy in rheumatic diseases: state-of-theart”; in Current Opinion Rheumatology.
- Erlandsson, L, Blumenthal, R, Eloranta, ML, Engel, H, Alm, G, Weiss, S, Leanderson, T (1998); “Interferon-beta is required for interferon-alpha production in mouse fibroblasts”; in Curr Biol.; Vol. 8; Issue 4;223-226.
- Taniguchi T, Takaoka A (2002); “The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors”; in Current Opinions in Immunology; Vol. 14; Issue 1; 111-116.
- Jegalian AG, Facchetti F, Jaffe ES (2009); “Plasmacytoid dendritic cells: physiologic roles and pathologic states”; in Advances in Anat Pathol; Vol.16; Issue 6; 392-404.
- Fitzgerald-Bocarsly P, Dai J, Singh S (2008); “Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history”; in Cytokine Growth Factor Rev; Vol. 19; Issue 1; 3-19.
- Li L, Sherry B (2010); “IFN-alpha expression and antiviral effects are subtype and cell type specific in the cardiac response to viral infection”; in Virology; Vol. 396; Issue 1; 59-68.
- Baig E, Fish EN (2008); “Distinct signature type I interferon responses are determined by the infecting virus and the target cell”; in Antivir Ther.; Vol. 13; Issue 3; 409-22.
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T (2006); “Neurons produce type I interferon during viral encephalitis”; in Proc Natl Acad Sci USA.; Vol. 103; Issue 20; 7835-7840.